Nitrogen in the Atmosphere: Why Can’t Plants and Animals Use It Directly? Short Response
The air we breathe is about 78% nitrogen gas, an abundant element essential for life. Yet, plants and animals can’t directly utilize this atmospheric nitrogen. Why is that? In short, atmospheric nitrogen (N2) is in a form that is chemically inert due to its strong triple bond. This article delves into the fascinating reasons why plants and animals cannot directly use atmospheric nitrogen, exploring the intricacies of nitrogen fixation and the crucial roles of microorganisms in making this vital element accessible to life.
The Inert Nature of Atmospheric Nitrogen (N2)
The primary reason plants and animals can’t directly use nitrogen from the air lies in the chemical structure of atmospheric nitrogen. Nitrogen exists as a diatomic molecule (N2), meaning two nitrogen atoms are bonded together. What makes this molecule so stable is the triple bond connecting the two nitrogen atoms. This triple bond is incredibly strong and requires a significant amount of energy to break. This high bond energy renders atmospheric nitrogen largely unreactive or inert under normal environmental conditions.
Think of it like this: Imagine trying to break apart two incredibly strong magnets stuck together. It takes a lot of force. Similarly, breaking the triple bond in N2 requires a substantial energy input that most plants and animals simply cannot generate through their normal metabolic processes. This inherent stability is why N2 is so prevalent in the atmosphere – it doesn’t readily react with other elements.
The Nitrogen Cycle: A Necessary Transformation
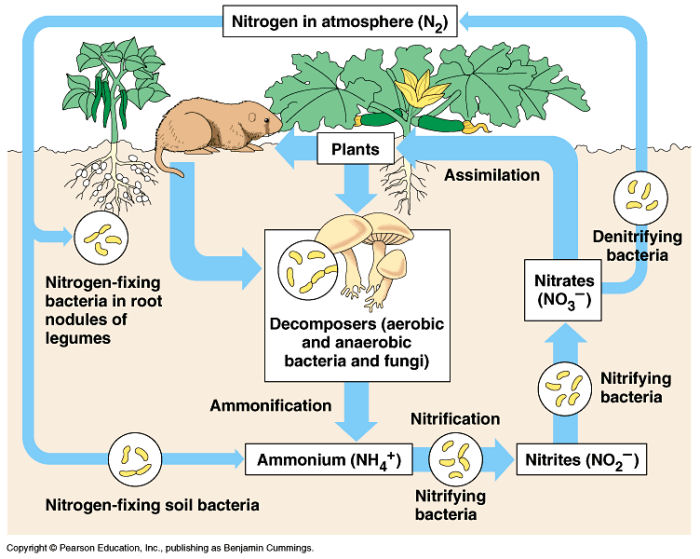
Because plants and animals can’t use atmospheric nitrogen directly, a process called the nitrogen cycle is essential for life on Earth. This cycle involves a series of transformations that convert atmospheric nitrogen into forms that plants can absorb and utilize, primarily ammonia (NH3), ammonium (NH4+), and nitrate (NO3–).
The nitrogen cycle is a complex interplay of biological, chemical, and physical processes. It includes nitrogen fixation, nitrification, assimilation, ammonification, and denitrification. Each step is crucial in converting nitrogen from one form to another, making it available to living organisms and returning it to the atmosphere.
Nitrogen Fixation: The Key to Unlocking Atmospheric Nitrogen
Nitrogen fixation is the process that converts atmospheric nitrogen (N2) into ammonia (NH3), a form of nitrogen that plants can eventually use. This process is primarily carried out by certain microorganisms, including bacteria and archaea. These nitrogen-fixing microorganisms possess a unique enzyme called nitrogenase, which catalyzes the reduction of N2 to NH3.
There are several types of nitrogen fixation:
- Biological Nitrogen Fixation: This is the most significant type of nitrogen fixation, performed by free-living bacteria (e.g., Azotobacter, Clostridium) and symbiotic bacteria (e.g., Rhizobium associated with legumes).
- Industrial Nitrogen Fixation: The Haber-Bosch process is an industrial method that uses high pressure and temperature to convert N2 to NH3. This process is used to produce synthetic fertilizers.
- Atmospheric Nitrogen Fixation: Lightning strikes can provide enough energy to break the triple bond in N2, allowing it to react with oxygen to form nitrogen oxides, which are then converted to nitrates.
Symbiotic Nitrogen Fixation: A Mutually Beneficial Relationship
Symbiotic nitrogen fixation is a particularly important process, especially in agricultural systems. The best-known example is the association between Rhizobium bacteria and leguminous plants (e.g., beans, peas, soybeans). These bacteria colonize the roots of legumes, forming nodules where nitrogen fixation occurs. The plant provides the bacteria with carbohydrates, and the bacteria provide the plant with ammonia. This symbiotic relationship allows legumes to thrive in nitrogen-poor soils.
Nitrification: Converting Ammonia to Nitrate
Once ammonia (NH3) is produced through nitrogen fixation, it is often converted to nitrate (NO3–) through a process called nitrification. Nitrification is a two-step process carried out by two different groups of bacteria:
- Ammonia Oxidation: Nitrosomonas bacteria convert ammonia (NH3) to nitrite (NO2–).
- Nitrite Oxidation: Nitrobacter bacteria convert nitrite (NO2–) to nitrate (NO3–).
Nitrate is a highly mobile form of nitrogen in the soil and is readily absorbed by plant roots. However, nitrate is also susceptible to leaching and denitrification, which can lead to nitrogen loss from the ecosystem.
Assimilation: Incorporating Nitrogen into Biomolecules
Assimilation is the process by which plants and animals incorporate inorganic nitrogen (ammonia, ammonium, or nitrate) into organic molecules, such as amino acids, proteins, and nucleic acids. Plants absorb nitrogen from the soil through their roots and use it to synthesize these essential biomolecules. Animals obtain nitrogen by consuming plants or other animals.
The assimilation of nitrogen is a critical step in the nitrogen cycle, as it allows nitrogen to be incorporated into the food chain and become available to all living organisms.
Ammonification: Decomposing Organic Matter
Ammonification is the process by which organic nitrogen (e.g., in dead plants and animals, or in animal waste) is converted back into ammonia (NH3) or ammonium (NH4+). This process is carried out by decomposers, such as bacteria and fungi, which break down organic matter and release ammonia as a waste product.
Ammonification is an important step in the nitrogen cycle, as it returns nitrogen from organic matter back into the soil, where it can be used by plants or converted to other forms through nitrification.
Denitrification: Returning Nitrogen to the Atmosphere
Denitrification is the process by which nitrate (NO3–) is converted back into atmospheric nitrogen (N2) or nitrous oxide (N2O). This process is carried out by denitrifying bacteria under anaerobic conditions (e.g., in waterlogged soils). Denitrification is an important process in the nitrogen cycle, as it removes excess nitrogen from the soil and returns it to the atmosphere.
However, denitrification can also have negative consequences, as it can lead to the loss of valuable nitrogen from agricultural systems. Furthermore, nitrous oxide (N2O) is a potent greenhouse gas that contributes to climate change.
The Role of Microorganisms: Nature’s Nitrogen Processors
Microorganisms are the unsung heroes of the nitrogen cycle. Without these tiny organisms, plants and animals would not be able to access the nitrogen they need to survive. Nitrogen-fixing bacteria, nitrifying bacteria, and denitrifying bacteria all play crucial roles in converting nitrogen from one form to another, making it available to living organisms and returning it to the atmosphere.
The activity of these microorganisms is influenced by a variety of factors, including soil pH, temperature, moisture, and the availability of other nutrients. Understanding these factors is essential for managing nitrogen cycling in agricultural and natural ecosystems.
Human Impact on the Nitrogen Cycle
Human activities have significantly altered the nitrogen cycle, primarily through the use of synthetic fertilizers, the cultivation of nitrogen-fixing crops, and the burning of fossil fuels. These activities have led to a dramatic increase in the amount of reactive nitrogen in the environment, with significant consequences for air and water quality, human health, and climate change.
The Haber-Bosch process, which is used to produce synthetic fertilizers, has allowed us to greatly increase agricultural productivity. However, the excessive use of nitrogen fertilizers can lead to nitrogen pollution, including:
- Eutrophication: Excess nitrogen in waterways can lead to algal blooms, which deplete oxygen and harm aquatic life.
- Groundwater Contamination: Nitrate from fertilizers can leach into groundwater, contaminating drinking water supplies.
- Air Pollution: Nitrogen oxides from fertilizers can contribute to smog and acid rain.
It is crucial to manage nitrogen use more sustainably to minimize these negative impacts. This includes using nitrogen fertilizers more efficiently, promoting the use of nitrogen-fixing crops, and reducing nitrogen emissions from industrial and agricultural sources.
Sustainable Nitrogen Management: A Path Forward
Sustainable nitrogen management is essential for ensuring food security, protecting the environment, and mitigating climate change. This involves adopting practices that minimize nitrogen losses, maximize nitrogen use efficiency, and reduce nitrogen pollution. Some key strategies include:
- Precision Agriculture: Using technology to apply nitrogen fertilizers only where and when they are needed.
- Cover Cropping: Planting cover crops to absorb excess nitrogen and prevent it from leaching into waterways.
- Integrated Nutrient Management: Combining organic and inorganic sources of nitrogen to optimize nutrient availability and minimize environmental impacts.
- Reducing Food Waste: Reducing food waste can decrease the overall demand for nitrogen fertilizers.
The Future of Nitrogen Use: Innovation and Adaptation
The future of nitrogen use will likely involve a combination of technological innovation and adaptive management strategies. Scientists are exploring new ways to improve nitrogen fixation efficiency, develop nitrogen-efficient crops, and reduce nitrogen emissions from agricultural and industrial sources. Farmers and policymakers will need to adapt their practices and policies to address the challenges posed by nitrogen pollution and climate change.
Understanding Nitrogen’s Unavailability: A Summary
In summary, the reason plants and animals cannot directly use atmospheric nitrogen is due to the strong triple bond in the N2 molecule, which makes it chemically inert. The nitrogen cycle, driven primarily by microorganisms, is essential for converting atmospheric nitrogen into forms that plants can absorb and utilize. Human activities have significantly altered the nitrogen cycle, leading to nitrogen pollution and other environmental problems. Sustainable nitrogen management practices are needed to ensure food security, protect the environment, and mitigate climate change. By understanding the intricacies of the nitrogen cycle and the role of microorganisms in nitrogen fixation, we can work towards a more sustainable future for nitrogen use.
